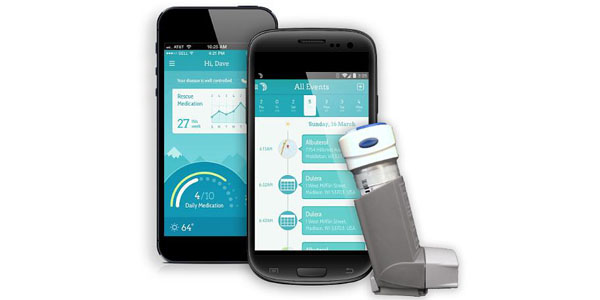
The latest version of the Propeller Health sensor has cleared through the FDA, the company announced Tuesday.
According to a release, the sensor is now 30 percent smaller, features a new collar that removes the need for adapter caps and eliminates the need for charging, increasing battery life to more than 18 months.
“We are delighted to share this news on World Asthma Day (May 6),” CEO David Van Sickle said in a statement. “We remain focused on building ever better tools to passively collect timely data about inhaled medication use, provide people with coaching and guidance that is relevant, personalized and actionable, and make care teams more efficient through new opportunities to intervene.”
Propeller helps patients and their physicians better understand and control asthma, Chronic Obstructive Pulmonary Disease (COPD) and other respiratory diseases. The FDA clearance allows the system to be used to help predict exacerbations in patients with asthma and COPD.
Propeller Health changed its name from Asthmapolis in September.

